Neuropathic pain is pain caused by a lesion or disease of the somatosensory system1 and is often described as sensations of burning, tingling, shooting, sharpness, stabbing, or like electrical shocks. It is associated with conditions seen in many hospice patients including cancer, diabetes, and stroke. Neuropathic pain may also be a complication of neuromuscular diseases (like ALS or multiple sclerosis) or nerve injury that may occur following trauma, surgery, or infection (e.g. shingles). Sometimes the cause may be unknown, but what’s clear is that neuropathic pain can be a significant source of discomfort and debility.
One group of drugs, collectively referred to as gabapentinoids, have become useful tools for the hospice clinician, specifically gabapentin (Neurontin) and pregabalin (Lyrica). Originally approved for treating seizures, they are probably better known as first-line agents for treating neuropathic pain.1,3,4 They have also been used to treat a variety of non-pain conditions including anxiety, refractory cough, hiccups, pruritus, and restless leg syndrome.3,5
Gabapentin Vs. Pregabalin
Gabapentin and pregabalin are both similar in structure and mechanism of action. They are both structurally similar to gamma aminobutyric acid (GABA), an inhibitory neurotransmitter. However, neither drug has activity in GABAergic neuronal systems.6 Both drugs bind to the alpha-2-delta subunit of voltage-gated calcium channels in the central nervous system and modulate the influx of calcium, inhibiting the release of excitatory neurotransmitters.3,5,6 The key differences between these two drugs lie in their pharmacokinetic and pharmacodynamic properties.
Compared to gabapentin, pregabalin exhibits more complete and rapid absorption, with a rate of absorption approximately 3 times greater than gabapentin’s.6 There are a couple of explanations for this. First, gabapentin is primarily absorbed in the small intestine, while pregabalin is absorbed at multiple sites, the small intestine and the ascending portion of the colon.6 Second, gabapentin’s absorption is saturable; meaning that as gabapentin doses increase, the rate of absorption and resulting bioavailability decreases. A study examined a gabapentin dose of 100mg given every 8 hours, which demonstrated a bioavailability of approximately 80%, however, when the dose was increased to 1600mg every 8 hours, the average bioavailability decreased to 27%.6 Pregabalin, on the other hand, averaged a bioavailability greater than or equal to 90% across a dose range of 75-900 mg/day.6
In addition to superior absorption, pregabalin achieves peak plasma concentrations faster than gabapentin. Gabapentin’s time to reach maximum plasma drug concentration (Tmax) is dose dependent. Low doses, like 100mg, reach Tmax approximately 1.7 hours after administration.6 As doses increase, Tmax increases to 3 to 4 hours post administration.6 Pregabalin has a much shorter Tmax, averaging less than 1 hour following a single dose up to 300 mg.6
Gabapentin exhibits non-linear pharmacokinetics, meaning that plasma concentrations don’t increase proportionally with increased doses. This presents a challenge, leading to a variable dose-response relationship. Pregabalin, however, exhibits linear pharmacokinetics, which leads to a more predictable dose-response relationship, as plasma concentrations increase proportionally with increasing doses.
In addition to pharmacokinetic differences, there are also pharmacodynamic differences between the two drugs. Pregabalin has a higher affinity for the alpha-2-delta subunit making it a more potent analgesic for neuropathic pain.7 Pregabalin also demonstrates improved pain reduction as doses are increased up to 600mg/day.6 Gabapentin, on the other hand, begins to exhibit a plateau effect in pain reduction once doses reach 1,800mg/day.6
Another advantage of pregabalin is that it may also offer simplified dosing. It is typically given two to three times per day. On the contrary, gabapentin is usually dosed three times daily and multiple capsules or tablets may be needed to achieve a specific dose. While both drugs are titrated to effect based on patient response and tolerability, pregabalin may allow for a quicker titration. The recommended titration period for pregabalin is over a period of several days to weeks and gabapentin is recommended to be titrated over a period of weeks to months.8
What Does the Literature Say?
There are limited studies directly comparing the efficacy of gabapentin to pregabalin. Based on the few studies examined, both drugs improve neuropathic pain but there is no clear difference between the two when it comes to pain reduction. However, pregabalin seems to result in quicker pain relief than gabapentin, which may be advantageous.
Ghosh et al.9
- A randomized, blinded, fixed dose study that compared the efficacy of gabapentin and pregabalin in a population of 100 patients with neuropathic pain.
- There were significant reductions in visual analog scale (VAS) scores, a subjective measurement for acute and chronic pain, for both the gabapentin and pregabalin groups at 0-4 weeks, 0-8 weeks, and 2-8 weeks indicating that both drugs are effective for pain reduction.
- No significant differences in pain reduction between the gabapentin and pregabalin groups.
- It took twice as long for gabapentin to achieve a significant reduction in pain quality assessment scores (PQAS). It took gabapentin 8 weeks to significantly reduce PQAS, while it took pregabalin 4 weeks to achieve a significant reduction.
- Concluded that while both drugs reduced pain, there was a greater reduction in the quality of pain perceived with pregabalin use.
Toth10
- A cohort study that examined reduction in pain severity with pregabalin substitution in patients who were gabapentin responders (defined as ≥30% neuropathic pain relief on the VAS scale) or non-responders at 0, 6, and 12 months and compared pain severity with patients who remained on gabapentin.
- Both gabapentin responders and non-responders achieved additional neuropathic pain relief of about 25% following substitution of pregabalin after 6 and 12 months.
- Improvement in pain scores was not seen in patients who remained on gabapentin.
- Patients in the gabapentin non-responder group were more likely to experience adverse effects like sedation, dizziness, and fatigue. Although, neither group experienced any serious adverse effects.
Devi et al.11
- A prospective, randomized, open label, 12-week study that compared the safety and efficacy of gabapentin, duloxetine, and pregabalin in 152 patients with painful diabetic peripheral neuropathy.
- Each drug demonstrated a significant reduction in VAS pain scores from baseline over the 12-week period with no significant difference between groups.
- Reduction in VAS score was achieved sooner in the pregabalin treatment group when compared to the gabapentin and duloxetine groups.
Roberson et al.12
- A prospective, single center, double-blind, randomized crossover trial that examined the effectiveness of pregabalin compared to gabapentin in the treatment of chronic sciatica in 18 patients over an 8-week treatment period.
- Participants were randomly assigned to be treated with gabapentin 400-800mg three times daily then pregabalin 150-300mg twice daily or vice versa.
- Each treatment period lasted 8 weeks and the crossover was completed after a one-week washout period.
- Concluded that the effects of both pregabalin and gabapentin were significant; however, gabapentin was superior in reducing pain intensity and was associated with fewer and less severe adverse effects.
- Several limitations:
- Limited study population – 18 patients with chronic sciatica.
- Duration of the treatment period for each medication was only 8 weeks, which may or may not be sufficient to test efficacy.
- Doses were initiated at significantly higher levels than in standard practice and were not consistent with equivalencies published in other studies.
- Patients were allowed to continue taking other existing analgesics that they were using prior to entering the study.
Lyrica Goes Generic
In July 2019, the U.S. Food and Drug administration (FDA) announced that it was granting approval for nine pharmaceutical companies to begin manufacturing generic Lyrica. Previously, Lyrica was a brand-only product, which translated to cost being a significant limitation for its use, especially in the hospice community. Since going generic, the price of pregabalin has decreased; however, it still remains relatively expensive. Currently, the average wholesale price (AWP) of pregabalin ranges from $7.18 per dose to $8.89 per dose.14 The AWP of Lyrica is $9.82 per dose.14 Only time will tell if the introduction of more competition into the market place will continue to drive down the AWP of pregabalin.
Converting to Pregabalin
Conversion from gabapentin to pregabalin or vice versa seems like a daunting task. However, there are a few studies examining such conversions. It is important to note that the studies specifically examined the conversion of gabapentin to pregabalin and the bi-directionality of this conversion was not investigated. As a result, clinical judgement should be used in these instances, as occasionally a more conservative approach may be warranted.
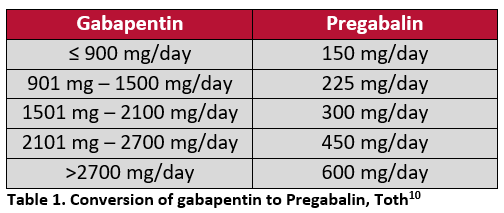
In the previously mentioned cohort study by Toth, a direct conversion was completed overnight; gabapentin was stopped after the evening dose and pregabalin was initiated the following morning using a conversion method proposed by the author (Table 1).10
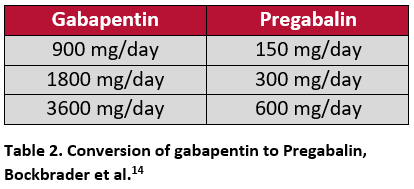
A modeling study by Bockbrader et al. assessed different conversion methods based on the pharmacokinetic profiles of gabapentin and pregabalin. This may be used as a guide for clinicians when transitioning patients between the two. The first proposed conversion method was similar to the method used in the Toth study, where gabapentin was immediately discontinued and pregabalin was initiated at the next scheduled dose. The second method proposed was a cross-titration where 50% of the existing gabapentin dose was co-administered with 50% of the new pregabalin dose for four days, then the full pregabalin dose was initiated and gabapentin was completely discontinued.15 The transitions were studied at three dosages using a 6:1 conversion (Table 2). The authors reported a seamless and rapid transition with the predicted pregabalin-equivalent concentrations being highly comparable with plasma pregabalin concentrations within one day in both groups.15 The study concluded that switching patients from gabapentin to pregabalin could be achieved by either of the two proposed methods.15
Abrupt discontinuation of gabapentin is not recommended when being used for the treatment of partial seizures. When switching patients with a seizure disorder from gabapentin to pregabalin, the dose should be reduced gradually over one week by gradually tapering down the daily dosage to minimize the risk of increased seizure frequency.1
Adverse Effects
Despite each medication being seemingly well tolerated, adverse effects are possible. The most common reported side effects of both medications include dizziness and somnolence with the latter being the most frequent reason for discontinuation. Both drugs can also cause peripheral edema and nausea.3,5Gabapentin may also cause ataxia and diarrhea and additional side effects of pregabalin include headache, dry mouth, vision changes, and constipation.3,5
Hospice Takeaways
Traditionally, gabapentin has been the preferred gabapentinoid in the hospice setting, primarily due to generic availability and cost. At this time, it’s difficult to determine whether or not pregabalin will become the gabapentinoid of choice. Pregabalin offers key advantages over gabapentin, including improved absorption, faster onset / titration, more predictable dose-response relationship, and more convenient dosing. While the prospect of reduced drug costs with generic pregabalin is encouraging, it remains more expensive than gabapentin — the most significant roadblock in advocating for preferential use of pregabalin over gabapentin. In the hospice setting, consider pregabalin as an option on a patient by patient basis, especially in those patients where pregabalin’s advantages over gabapentin make it an attractive alternative. Simultaneously, it is also reasonable to explore substitution of gabapentin in place of pregabalin to control drug costs!
Written by:
Kelly Tocki, PharmD Candidate 2020, University of Iowa
Reviewed by:
John Corrigan, PharmD
Clinical Pharmacist, OnePoint Patient Care
References
- Kremer M, Salvat E, Muller A, et al. Antidepressants and Gabapentinoids in Neuropathic Pain: Mechanistic Insights. Neuroscience. 2016; 338: 183-206
- Neurontin [package insert]. U.S. Food and Drug Administration Website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf. Revised October 2017. Accessed December 28,2020.
- Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Accessed December 28, 2020.
- Lyrica [package insert]. U.S. Food and Drug Administration Website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022075s000lbl.pdf. Revised June 2019. Accessed December 28, 2020.
- Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Accessed December 28, 2020.
- Bockbrader H, Wesche D, Miller R, et al. A Comparison of the Pharmacokinetics and Pharmacodynamics of Pregabalin and Gabapentin. Clin Pharmacokinet. 2010; 49(10): 661-669.
- Fundin, J. How gabapentin differs from pregabalin. Pharmacy Times. 2015. https://www.pharmacytimes.com/contributor/jeffrey-fudin/2015/09/how-gabapentin-differs-from-pregabalin?p=1. Accessed September 16, 2019.
- Clinical Resource, Comparison of Gabapentin and Pregabalin. Pharmacist’s Letter/Prescriber’s Letter. August 2018.
- Ghosh AK, Ghosh A, Kundu A, Das AK, Bhattacharya KB. Comparative study of efficacy and safety of pregabalin and gabapentin in neuropathic pain. Asian J Pharm Life Sci. 2012;2:64-71.
- Toth C, Substitution of Gabapentin Therapy with Pregabalin Therapy in Neuropathic Pain due to Peripheral Neuropathy, Pain Medicine. 2010;11(30):456-465. https://doi.org/10.1111/j.1526-4637.2009.00796.x
- Devi P, Madhu K, Ganapathy B, Sarma G, John L, Kulkarni C. Evaluation of efficacy and safety of gabapentin, duloxetine, and pregabalin in patients with painful diabetic peripheral neuropathy. Indian J Pharmacol. 2012;44(1):51-6.
- Roberson K, Marshman L, Plummer D, et al. Effect of Gabapentin vs Pregabalin on Pain Intensity in Adults with Chronic Sciatica. JAMA Neurol. 2019;76(1):28-34.
- FDA approves first generics of lyrica [press release]. White Oak, MD: FDA; Published July 22, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-generics-lyrica. Accessed September 16, 2019
- AmerisourceBergen
- Bockbrader HN, Budhwani MN, Wesche DL. Gabapentin to pregabalin therapy transition: a pharmacokinetic simulation. Am J Ther. 2013;20(1):32-6.
