We hope you were able to join the March and April presentations from our 2021 clinical webinar series, Budget Busters – Best Practices with NOACs, Insulins, and COPD Drugs in Hospice. Throughout the webinar, our Senior Clinical Pharmacist, Melissa Corak presented pearls and practical strategies to promote cost avoidance in circumstances where hospices would otherwise be on the hook for paying for these often high cost medications. If you didn’t have the opportunity to tune-in (or want a refresher), the webinar can still be viewed on-demand on OneConnectPoint under the Clinical Resources tab.
In just a few short months since the webinar’s debut, we’ve seen some exciting developments in the insulin and COPD medication landscape.
Specifically, an existing insulin glargine product has been approved as an interchangeable biosimilar and two generic nebulized long-acting beta agonists (LABAs) have come to market (one of them a few months earlier than expected).
Semglee (insulin glargine-yfgn) – a substitute for Lantus
In July 2021, the U.S. Food and Drug Administration (FDA) approved Semglee (insulin glargine-yfgn) as the first interchangeable biosimilar insulin.1,2 Being biosimilar means there are no clinically meaningful differences in terms of efficacy, safety, purity, or potency between Semglee and Lantus (the reference product).3-5 And now that it’s been approved as interchangeable with Lantus, pharmacists will soon be able to automatically substitute Semglee unless the prescriber indicates brand Lantus is necessary — very similar to how generic drugs are substituted for brand name drugs.3-4,6
The Semglee products on the market today aren’t interchangeable with Lantus just yet.7 New Semglee packaging will have a suffix (“-yfgn”) added to the nonproprietary name (insulin glargine).7 This will help differentiate it from versions manufactured before its approval as an interchangeable biosimilar. As a naming convention for interchangeable biosimilars, the FDA requires that a meaningless, lowercased four-letter suffix be applied to the product’s nonproprietary name.8 The addition of a suffix to the nonproprietary name is intended to primarily serve as a way to differentiate between products — this is used to help with post market surveillance, adverse event tracking, and improve patient safety.8Interchangeable Semglee should be available before the end of the year.7,9 In the meantime, our pharmacists will continue to recommend Semglee as a less expensive alternative to Lantus, but will have to call prescribers first.
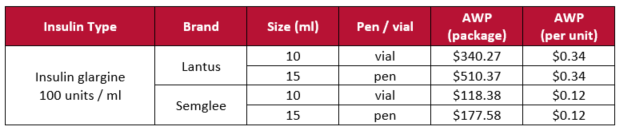
Based on current pricing (Table 1), up to 65% savings can be realized by using Semglee in place of Lantus and we could potentially see even more savings if and when other insulin glargine biosimilars make it to market.10 But, don’t expect other manufacturers to throw their hats in the ring just yet… Viatris and their partner (Biocon Biologics) have exclusive marketing rights for 12 months (from the date of commercial launch) before the FDA can approve another biosimilar that is interchangeable with Lantus.9
You can learn more about biosimilars and interchangeable products here.
Table 1. Lantus and Semglee pricing10
Brovana and Perforomist go generic
Arformoterol tartrate (Brovana) and formoterol fumarate (Perforomist) are nebulized LABAs used for maintenance treatment of bronchoconstriction in COPD patients.11 Both are dosed twice daily; this is an advantage over short-acting beta agonists (SABAs), like albuterol, which is usually given more frequently due to its shorter expected duration of action (3 to 6 hours) when given via nebulizer.11 Despite their advantages, Brovana and Peroformist’s use in the hospice setting has historically been limited by their high cost, the AWP of the branded products exceeds $1,200 per month.10 But, this may change with the release of new generic versions of these drugs.
Since June 2021, three manufacturers (Lupin, Cipla, and Glenmark) have launched their respective versions of arformoterol tartrate (generic Brovana) and two manufacturers (Teva and Mylan) have made formoterol fumarate (generic Perforomist) available.12-17 The listed AWP of the new generic LABAs is currently 5-10% lower compared to their branded counterparts.10 But, generic drugs typically receive more favorable contract pricing compared to brand name drugs. It’s typical to see minimal price reductions in the period immediately following initial generic releases; savings are likely to be more substantial as more generic manufacturers release competing products. Notably (as of now), two other manufacturers may enter the market; Akar Pharma has FDA approval and Teva has a tentative FDA approval for arformoterol tartrate.14,15
Takeaway:
The prospect of more biosimilar products and generic drugs hitting the market is certainly exciting. Increased competition and subsequent price reductions should be welcome news to hospices.
Written By:
John Corrigan, PharmD
References:
- Tucker M. FDA Approves First Interchangeable Biosimilar Insulin. Medscape. July 28, 2021. Accessed August 10, 2021.
- S. Food and Drug Administration. FDA Approves First Interchangeable Biosimilar Insulin Product for Treatment of Diabetes. July 28, 2021. Accessed August 10, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-interchangeable-biosimilar-insulin-product-treatment-diabetes
- S. Food and Drug Administration. Biosimilar and Interchangeable. October 23, 2017. Accessed August 10, 2021. https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products
- S. Food and Drug Administration. Prescribing Biosimilar and Interchangeable Products. October 23, 2017. Accessed August 10, 2021. https://www.fda.gov/drugs/biosimilars/prescribing-biosimilar-and-interchangeable-products
- Clinical Resource, FAQs About Biosimilars. Pharmacist’s Letter/Prescriber’s Letter. December 2018. Accessed August 17, 2021. https://pharmacist.therapeuticresearch.com/Content/Segments/PRL/2015/Oct/FAQs-About-Biosimilars-8977
- Insero A, Hagen T. FDA Approves Insulin Glargine as Country’s First Interchangeable Biosimilar. AJMC. July 29, 2021. Accessed August 10, 2021. https://www.ajmc.com/view/fda-approves-insulin-glargine-as-country-s-first-interchangeable-biosimilar
- Explain why Semglee is Now “Interchangeable” with Lantus. Pharmacist’s Letter/Prescriber’s Letter. August 13, 2021. Accessed August 17, 2021. https://pharmacist.therapeuticresearch.com/Content/Segments/PRL/2021/Aug/Explain-Why-Semglee-Is-Now-Interchangeable-With-Lantus-S2108006
- Gottlieb MD, S. Statement from FDA Commissioner Scott Gottlieb, M.D., on FDA’s steps on naming of biological medicines to balance competition and safety for patients receiving these Products. U.S. Food and Drug Administration. March 7, 2019. Accessed August 20, 2021. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-fdas-steps-naming-biological-medicines-balance
- Viatris Inc. Viatris Inc. and Biocon Biologics Receive Historic Approval for First Interchangeable Biosimilar Semglee (insulin glargine-yfgn) injection for the Treatment of Diabetes. July 28, 2021. Accessed August 11, 2021. https://newsroom.viatris.com/2021-07-28-Viatris-Inc-and-Biocon-Biologics-Receive-Historic-Approval-for-First-Interchangeable-Biosimilar-Semglee-R-insulin-glargine-yfgn-injection-for-the-Treatment-of-Diabetes
- AmerisourceBergen
- Wolters Kluwer Health, Inc. 2021. Accessed August 11, 2021.
- Anticipated Availability of First-Time Generics. Pharmacist’s Letter / Prescriber’s Letter. November 2020.
- Lupin Receives Tentative Approval for Arformoterol Tartrate Inhalation Solution 15mcg (base / 2ml. April 20, 2020. Accessed August 11, 2021. https://www.lupin.com/lupin-receives-tentative-approval-for-arformoterol-tartrate-inhalation-solution-15-mcg-base-2-ml/
- Lupin Launches Authorized Generic Version of Brovana in the United States. June 3, 2021. Accessed August 11, 2021. https://www.lupin.com/lupin-launches-authorized-generic-version-of-brovana-in-the-united-states/
- Seth A. After Lupin Launch, Cipla Introduces Brovana Rival. Generic Bulletin. June 29, 2021. Accessed August 9, 2021. https://generics.pharmaintelligence.informa.com/GB151046/After-Lupin-Launch-Cipla-Introduces-US-Brovana-Rival
- Teva Announces Its Launch of the First Generic Perforomist, Formoterol Fumarate Inhalation Solution, 20 mcg / 2 ml, Used to Treat Chronic Obstructive Pulmonary Disease (COPD in the United State. June 22, 2021. Accessed August 11, 2021. https://www.tevapharm.com/news-and-media/latest-news/teva-announces-its-launch-of-the-first-generic-perforomist-formoterol-fumarate-inhalation-solution-20-/
- Rudge D. Teva Capitalizes on Patent Expiry to Launch First Perforomist Rival. Generics Bulletin. June 24, 2021. Accessed August 9, 2021. https://generics.pharmaintelligence.informa.com/GB151032/Teva-Capitalizes-On-Patent-Expiry-To-Launch-First-Perforomist-Rival
